Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.
Topic Contents
- General Information About Ovarian Borderline Tumors
- Stages of Ovarian Borderline Tumors
- Treatment Option Overview
- Treatment of Early Stage Ovarian Borderline Tumors (Stages I and II)
- Treatment of Advanced Stage Ovarian Borderline Tumors (Stages III and IV)
- Treatment of Recurrent Ovarian Borderline Tumors
- To Learn More About Ovarian Borderline Tumors
- About This PDQ Summary
Ovarian Borderline Tumors Treatment (PDQ®): Treatment - Patient Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
General Information About Ovarian Borderline Tumors
Ovarian borderline tumor is a disease in which abnormal cells form in the tissue covering the ovary.
Ovarian borderline tumors have abnormal cells that may become cancer, but usually do not. This disease usually remains in the ovary. When disease is found in one ovary, the other ovary should also be checked carefully for signs of disease.
The ovaries are a pair of organs in the female reproductive system. They are in the pelvis, one on each side of the uterus (the hollow, pear-shaped organ where a fetus grows). Each ovary is about the size and shape of an almond. The ovaries make eggs and female hormones. 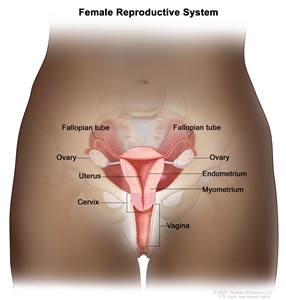
Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium.
Signs and symptoms of ovarian borderline tumor include pain or swelling in the abdomen.
Ovarian borderline tumor may not cause early signs or symptoms. If you do have signs or symptoms, they may include:
- pain or swelling in the abdomen
- pain in the pelvis
- gastrointestinal problems, such as gas, bloating, or constipation
These signs and symptoms may be caused by other conditions. If they get worse or do not go away on their own, check with your doctor.
Tests that examine the ovaries are used to diagnose and stage ovarian borderline tumor.
In addition to asking about your personal and family health history and doing a physical exam, your doctor may perform the following tests and procedures:
- Pelvic exam is an exam of the vagina, cervix, uterus, fallopian tubes, ovaries, and rectum. A speculum is inserted into the vagina and the doctor or nurse looks at the vagina and cervix for signs of disease. A Pap test of the cervix is usually done. The doctor or nurse also inserts one or two lubricated, gloved fingers of one hand into the vagina and places the other hand over the lower abdomen to feel the size, shape, and position of the uterus and ovaries. The doctor or nurse also inserts a lubricated, gloved finger into the rectum to feel for lumps or abnormal areas.
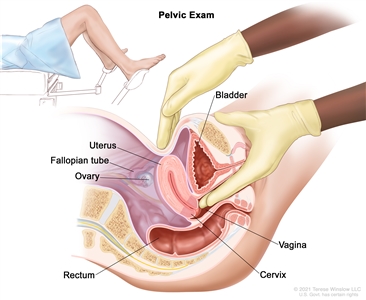
Pelvic exam. A doctor or nurse inserts one or two lubricated, gloved fingers of one hand into the vagina and presses on the lower abdomen with the other hand. This is done to feel the size, shape, and position of the uterus and ovaries. The vagina, cervix, fallopian tubes, and rectum are also checked. - Ultrasound exam is a procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. The picture can be printed to be looked at later.
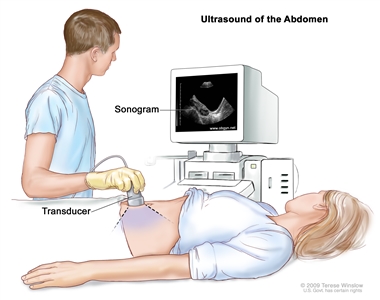
Abdominal ultrasound. An ultrasound transducer connected to a computer is passed over the surface of the abdomen. The ultrasound transducer bounces sound waves off internal organs and tissues to make echoes that form a sonogram (computer picture). Other patients may have a transvaginal ultrasound.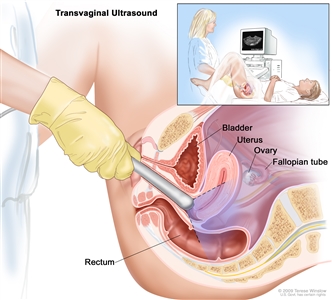
Transvaginal ultrasound. An ultrasound probe connected to a computer is inserted into the vagina and is gently moved to show different organs. The probe bounces sound waves off internal organs and tissues to make echoes that form a sonogram (computer picture). - CT scan (CAT scan) uses a computer linked to an x-ray machine to make a series of detailed pictures of areas inside the body. The pictures are taken from different angles and are used to create 3-D views of tissues and organs. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- CA 125 assay is a laboratory test that measures the level of CA 125 in the blood. CA 125 is a substance released by cells into the bloodstream. An increased CA 125 level is sometimes a sign of cancer or other condition.
- Chest x-ray is a type of radiation that can go through the body and make pictures of the organs and bones inside the chest.
- Biopsy is the removal of cells or tissues so they can be viewed under a microscope by a pathologist to check for signs of cancer. The tissue is usually removed during surgery to remove the tumor.
- Staging laparotomy is surgery to determine the extent of cancer within the abdomen. An incision (cut) is made in the wall of the abdomen to remove tissue so a pathologist can check it for signs of cancer.
Some people decide to get a second opinion.
You may want to get a second opinion to confirm your diagnosis and treatment plan. If you seek a second opinion, you will need to get medical test results and reports from the first doctor to share with the second doctor. The second doctor will review the pathology report, slides, and scans. They may agree with the first doctor, suggest changes or another treatment approach, or provide more information about your tumor.
Learn more about choosing a doctor and getting a second opinion at Finding Cancer Care. You can contact NCI's Cancer Information Service via chat, email, or phone (both in English and Spanish) for help finding a doctor, hospital, or getting a second opinion. For questions you might want to ask at your appointments, visit Questions to Ask Your Doctor About Cancer.
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on:
- the stage of the disease (whether it affects part of the ovary, involves the whole ovary, or has spread to other places in the body)
- what type of cells make up the tumor
- the size of the tumor
- the patient's general health
Patients with ovarian borderline tumors have a good prognosis, especially when the tumor is found early.
Stages of Ovarian Borderline Tumors
After ovarian borderline tumor has been diagnosed, tests are done to find out if abnormal cells have spread within the ovary or to other parts of the body.
Cancer stage describes the extent of cancer in the body, such as the size of the tumor, whether it has spread, and how far it has spread from where it first formed. It is important to know the stage of the ovarian borderline tumors to plan the best treatment. Most people are diagnosed with stage I disease.
Borderline ovarian tumor staging usually uses the FIGO staging system. The tumor may be described by this staging system in your pathology report. Based on the FIGO results, a stage (I, II, III, or IV, also written as 1, 2, 3, or 4) is assigned to your tumor. When talking to you about your diagnosis, your doctor may describe the tumor as one of these stages.
The following stages are used for ovarian borderline tumor:
Stage I (also called stage 1) ovarian borderline tumor
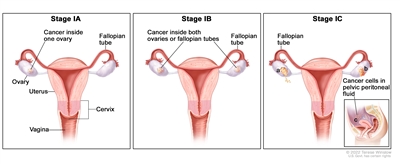
In stage IA, cancer is found inside a single ovary or fallopian tube. In stage IB, cancer is found inside both ovaries or fallopian tubes. In stage IC, cancer is found inside one or both ovaries or fallopian tubes and one of the following is true: (a) either the tumor or the capsule (outer covering) of the ovary has ruptured (broken open), or (b) cancer is also found on the surface of the ovary or fallopian tube, or (c) cancer cells are found in the pelvic peritoneal fluid.
In stage I, the tumor is found in one or both ovaries or fallopian tubes. Stage I is divided into stage IA, stage IB, and stage IC.
- Stage IA: The tumor is found inside a single ovary or fallopian tube.
- Stage IB: The tumor is found inside both ovaries or fallopian tubes.
- Stage IC: The tumor is found inside one or both ovaries or fallopian tubes and one of the following is true:
- tumor cells are found on the outside surface of one or both ovaries or fallopian tubes; or
- the capsule (outer covering) of the ovary ruptured (broke open) before or during surgery; or
- tumor cells are found in the fluid of the peritoneal cavity (the body cavity that contains most of the organs in the abdomen) or in washings of the peritoneum (tissue lining the peritoneal cavity).
Stage II (also called stage 2) ovarian borderline tumor
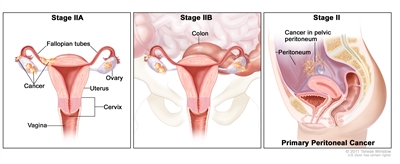
In stage IIA, cancer is found in one or both ovaries or fallopian tubes and has spread to the uterus and/or the fallopian tubes and/or the ovaries. In stage IIB, cancer is found in one or both ovaries or fallopian tubes and has spread to organs in the peritoneal cavity, such as the colon. In primary peritoneal cancer, cancer is found in the pelvic peritoneum and has not spread there from another part of the body.
In stage II, the tumor is found in one or both ovaries or fallopian tubes and has spread into other areas of the pelvis, or primary peritoneal cancer is found within the pelvis. Stage II is divided into stage IIA and stage IIB.
- Stage IIA: The tumor has spread from where it first formed to the uterus and/or the fallopian tubes and/or the ovaries.
- Stage IIB: The tumor has spread from the ovary or fallopian tube to organs in the peritoneal cavity (the space that contains the abdominal organs).
Stage III (also called stage 3) ovarian borderline tumor
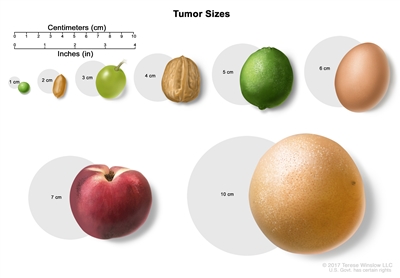
Tumor sizes are often measured in centimeters (cm) or inches. Common food items that can be used to show tumor size in cm include: a pea (1 cm), a peanut (2 cm), a grape (3 cm), a walnut (4 cm), a lime (5 cm or 2 inches), an egg (6 cm), a peach (7 cm), and a grapefruit (10 cm or 4 inches).
In stage III, the tumor is found in one or both ovaries or fallopian tubes, or is primary peritoneal cancer, and has spread outside the pelvis to other parts of the abdomen and/or to nearby lymph nodes. Stage III is divided into stage IIIA, stage IIIB, and stage IIIC.
- In stage IIIA, one of the following is true:
- The tumor has spread to lymph nodes in the area outside or behind the peritoneum only; or
- Tumor cells that can be seen only with a microscope have spread to the surface of the peritoneum outside the pelvis, such as the omentum (a fold of the peritoneum that surrounds the stomach and other organs in the abdomen). The tumor may have spread to nearby lymph nodes.
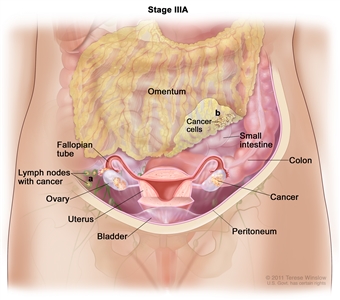
In stage IIIA, cancer is found in one or both ovaries or fallopian tubes and (a) cancer has spread to lymph nodes behind the peritoneum only, or (b) cancer cells that can be seen only with a microscope have spread to the surface of the peritoneum outside the pelvis, such as the omentum. Cancer may have also spread to nearby lymph nodes. - Stage IIIB: The tumor has spread to the peritoneum outside the pelvis, such as the omentum, and the tumor in the peritoneum is 2 centimeters or smaller. The tumor may have spread to lymph nodes behind the peritoneum.
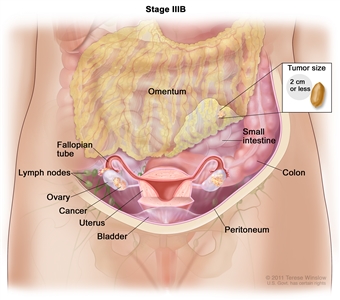
In stage IIIB, cancer is found in one or both ovaries or fallopian tubes and has spread to the peritoneum outside the pelvis, such as the omentum. The cancer in the omentum is 2 centimeters or smaller. Cancer may have also spread to lymph nodes behind the peritoneum. - Stage IIIC: The tumor has spread to the peritoneum outside the pelvis, such as the omentum, and the tumor in the peritoneum is larger than 2 centimeters. The tumor may have spread to lymph nodes behind the peritoneum or to the surface of the liver or spleen.
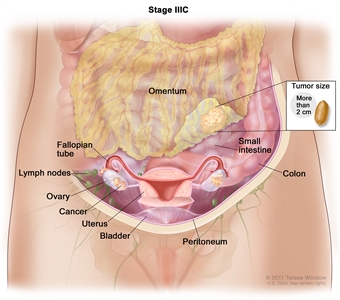
In stage IIIC, cancer is found in one or both ovaries or fallopian tubes and has spread to the peritoneum outside the pelvis, such as the omentum. The cancer in the omentum is larger than 2 centimeters. Cancer may have also spread to lymph nodes behind the peritoneum or to the surface of the liver or spleen (not shown).
Stage IV (also called stage 4) ovarian borderline tumor
In stage IV, tumor cells have spread beyond the abdomen to other parts of the body. Stage IV is divided into stage IVA and stage IVB. 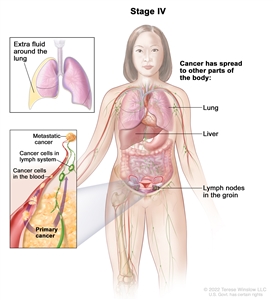
In stage IV, cancer has spread beyond the abdomen to other parts of the body. In stage IVA, cancer cells are found in extra fluid that builds up around the lungs. In stage IVB, cancer has spread to organs and tissues outside the abdomen, including the lung, liver, and lymph nodes in the groin.
- Stage IVA: Tumor cells are found in extra fluid that builds up around the lungs.
- Stage IVB: The tumor has spread to organs and tissues outside the abdomen, including lymph nodes in the groin.
Ovarian borderline tumors can recur (come back) after they have been treated.
Recurrent ovarian borderline tumors are tumors that have come back after they have been treated. The tumors may come back in the other ovary or in other parts of the body. Tests will be done to help determine where the tumor has returned. The type of treatment for a recurrent ovarian borderline tumor will depend on where it has come back.
Treatment Option Overview
There are different types of treatment for patients with ovarian borderline tumors.
Different types of treatments are available for ovarian borderline tumors. You and your care team will work together to decide your treatment plan, which may include more than one type of treatment. Many factors will be considered, such as the stage of the tumor, your overall health, and your preferences. Your plan will include information about your tumor, the goals of treatment, your treatment options and the possible side effects, and the expected length of treatment.
Talking with your care team before treatment begins about what to expect will be helpful. You'll want to learn what you need to do before treatment begins, how you'll feel while going through it, and what kind of help you will need. To learn more, visit Questions to Ask Your Doctor About Treatment.
The following types of treatment are used:
Surgery
The type of surgery (removing the tumor in an operation) depends on the size and spread of the tumor and the patient's plans for having children. Surgery may include:
- Unilateral salpingo-oophorectomy is surgery to remove one ovary and one fallopian tube.
- Bilateral salpingo-oophorectomy is surgery to remove both ovaries and both fallopian tubes.
- Total hysterectomy and bilateral salpingo-oophorectomy is surgery to remove the uterus, cervix, and both ovaries and fallopian tubes. If the uterus and cervix are taken out through the vagina, the operation is called a vaginal hysterectomy. If the uterus and cervix are taken out through a large incision (cut) in the abdomen, the operation is called a total abdominal hysterectomy. If the uterus and cervix are taken out through a small incision (cut) in the abdomen using a laparoscope, the operation is called a total laparoscopic hysterectomy.
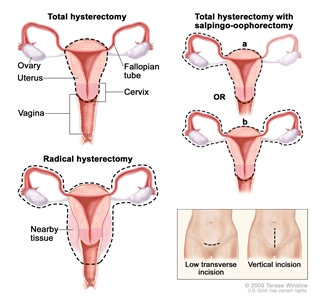
Hysterectomy. The uterus is surgically removed with or without other organs or tissues. In a total hysterectomy, the uterus and cervix are removed. In a total hysterectomy with salpingo-oophorectomy, (a) the uterus plus one (unilateral) ovary and fallopian tube are removed; or (b) the uterus plus both (bilateral) ovaries and fallopian tubes are removed. In a radical hysterectomy, the uterus, cervix, both ovaries, both fallopian tubes, and nearby tissue are removed. These procedures are done using a low transverse incision or a vertical incision. - Partial oophorectomy is surgery to remove part of one ovary or part of both ovaries.
- Omentectomy is surgery to remove the omentum (a tissue layer that lines the abdominal wall).
After the doctor removes all disease that can be seen at the time of the surgery, the patient may be given chemotherapy (also called chemo) after surgery to kill any tumor cells that are left. Treatment given after the surgery to lower the risk that the tumor will come back is called adjuvant therapy.
Chemotherapy
Chemotherapy uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. Chemotherapy for ovarian borderline tumors is usually systemic, meaning it is injected into a vein or given by mouth. When given this way, the drugs enter the bloodstream to reach cancer cells throughout the body.
Learn more about how chemotherapy works, how it is given, common side effects, and more at Chemotherapy to Treat Cancer and Chemotherapy and You: Support for People With Cancer.
New types of treatment are being tested in clinical trials.
For some people, joining a clinical trial may be an option. There are different types of clinical trials for people with cancer. For example, a treatment trial tests new treatments or new ways of using current treatments. Supportive care and palliative care trials look at ways to improve quality of life, especially for those who have side effects from cancer and its treatment.
You can use the clinical trial search to find NCI-supported cancer clinical trials accepting participants. The search allows you to filter trials based on the type of cancer, your age, and where the trials are being done. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Learn more about clinical trials, including how to find and join one, at Clinical Trials Information for Patients and Caregivers.
Treatment for ovarian borderline tumors may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Follow-up care may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).
Treatment of Early Stage Ovarian Borderline Tumors (Stages I and II)
Surgery is the standard treatment for early stage ovarian borderline tumors. The type of surgery usually depends on whether a patient plans to have children.
For patients who plan to have children, surgery is either:
- unilateral salpingo-oophorectomy or
- partial oophorectomy
To prevent recurrence of disease, most doctors recommend surgery to remove the remaining ovarian tissue when a patient no longer plans to have children.
For patients who do not plan to have children, treatment may be hysterectomy and bilateral salpingo-oophorectomy.
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Advanced Stage Ovarian Borderline Tumors (Stages III and IV)
Treatment for advanced stage ovarian borderline tumors may be hysterectomy, bilateral salpingo-oophorectomy, and omentectomy. A lymph node dissection may also be done. Patients with advanced disease should undergo a total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, node sampling, and aggressive cytoreductive surgery.
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
Treatment of Recurrent Ovarian Borderline Tumors
Treatment for recurrent ovarian borderline tumors may include:
- surgery to remove cancer that has spread in the abdominal cavity
- surgery followed by chemotherapy
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
To Learn More About Ovarian Borderline Tumors
For general cancer information and other resources from the National Cancer Institute, visit:
About This PDQ Summary
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government's center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of ovarian borderline tumors. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary]."
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Ovarian Borderline Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/ovarian/patient/ovarian-low-malignant-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389247]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's E-mail Us.
Last Revised: 2025-04-10
If you want to know more about cancer and how it is treated, or if you wish to know about clinical trials for your type of cancer, you can call the NCI's Cancer Information Service at 1-800-422-6237, toll free. A trained information specialist can talk with you and answer your questions.
This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content.
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.




